Nature Cell Biology. Nichols, R.J., et al. 2018.
Navire Pharma is developing SHP2 inhibitors as potentially effective additions to the therapeutic arsenal for difficult-to-treat cancers.
Science
SHP2
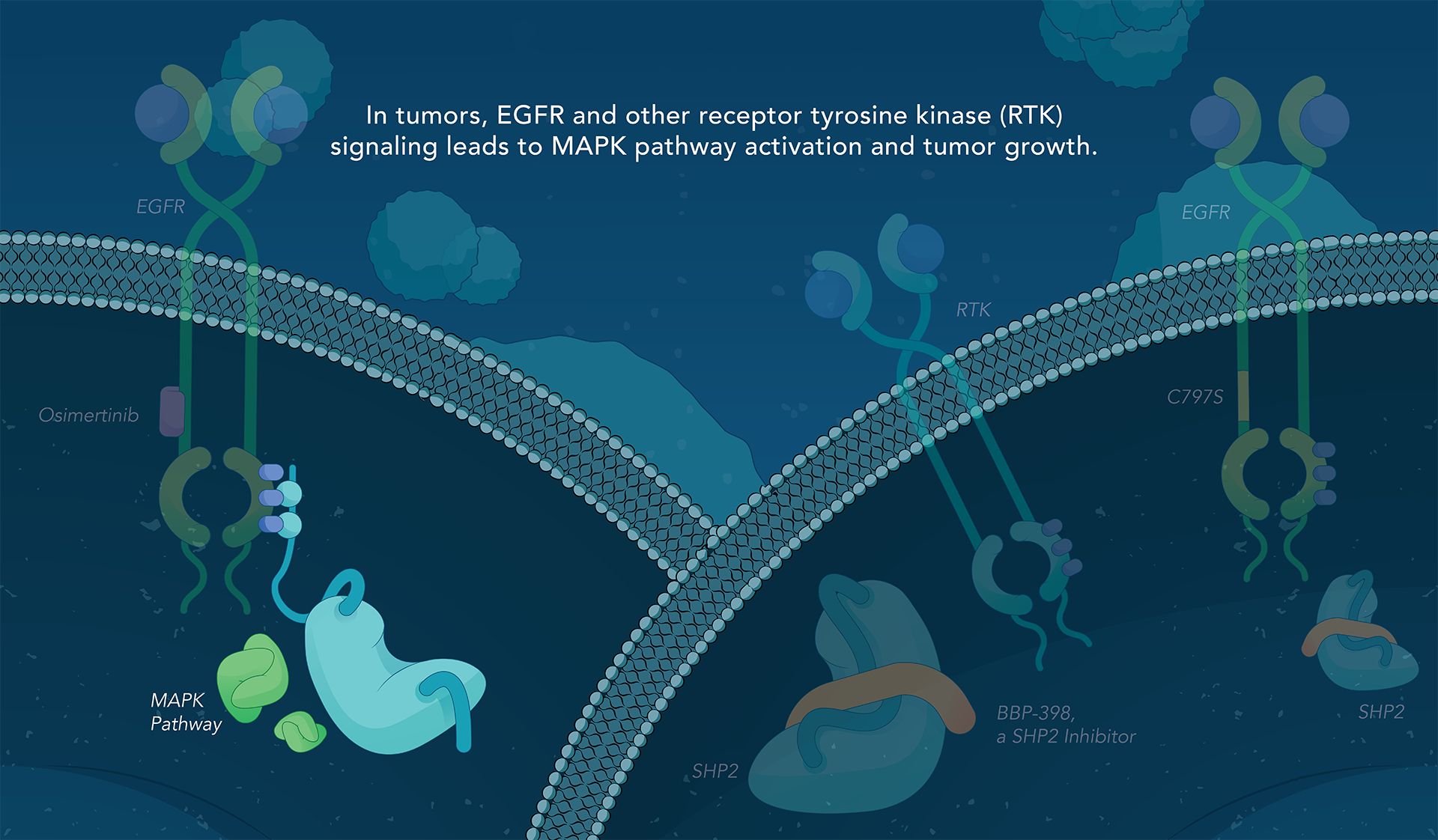
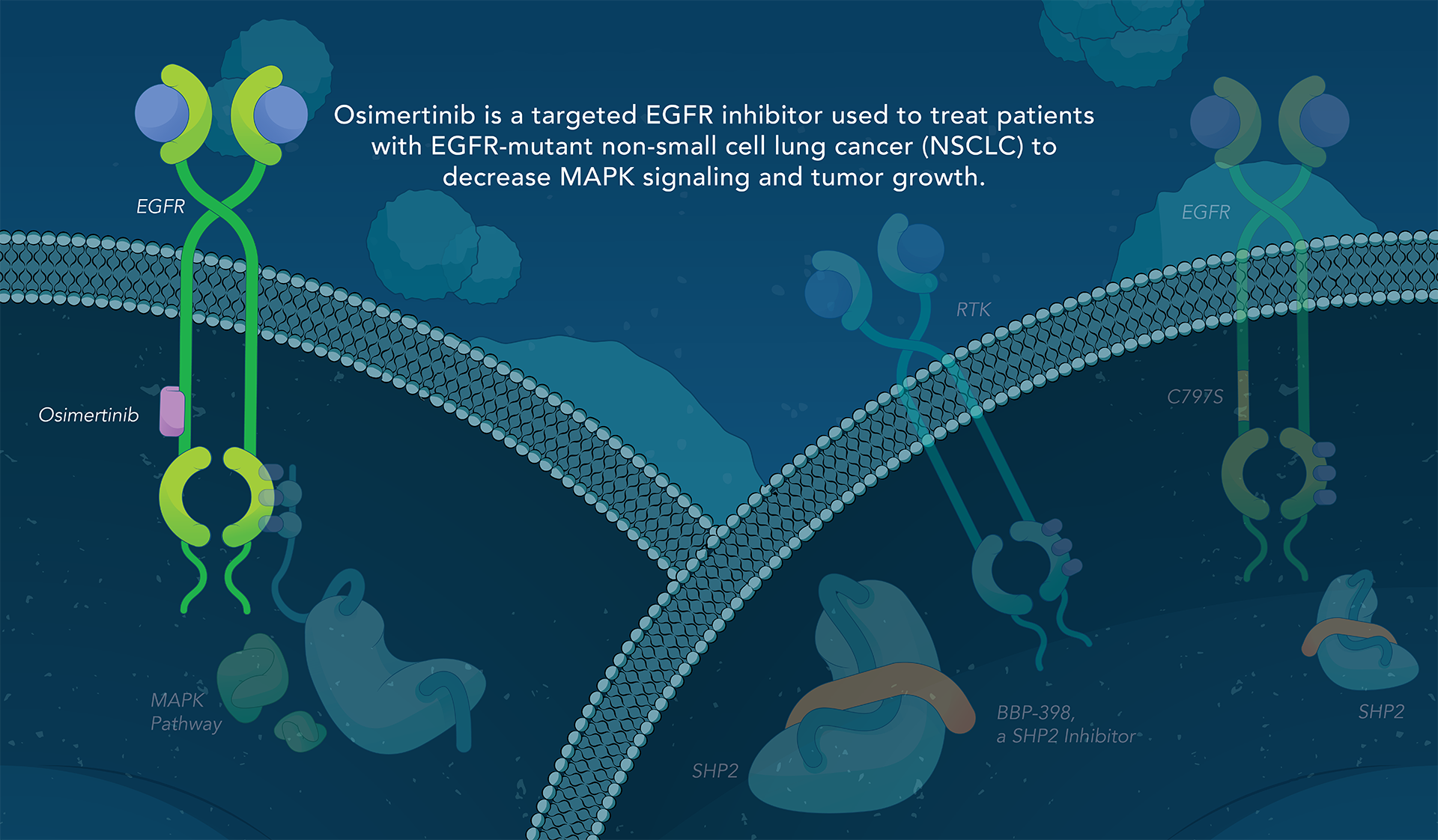
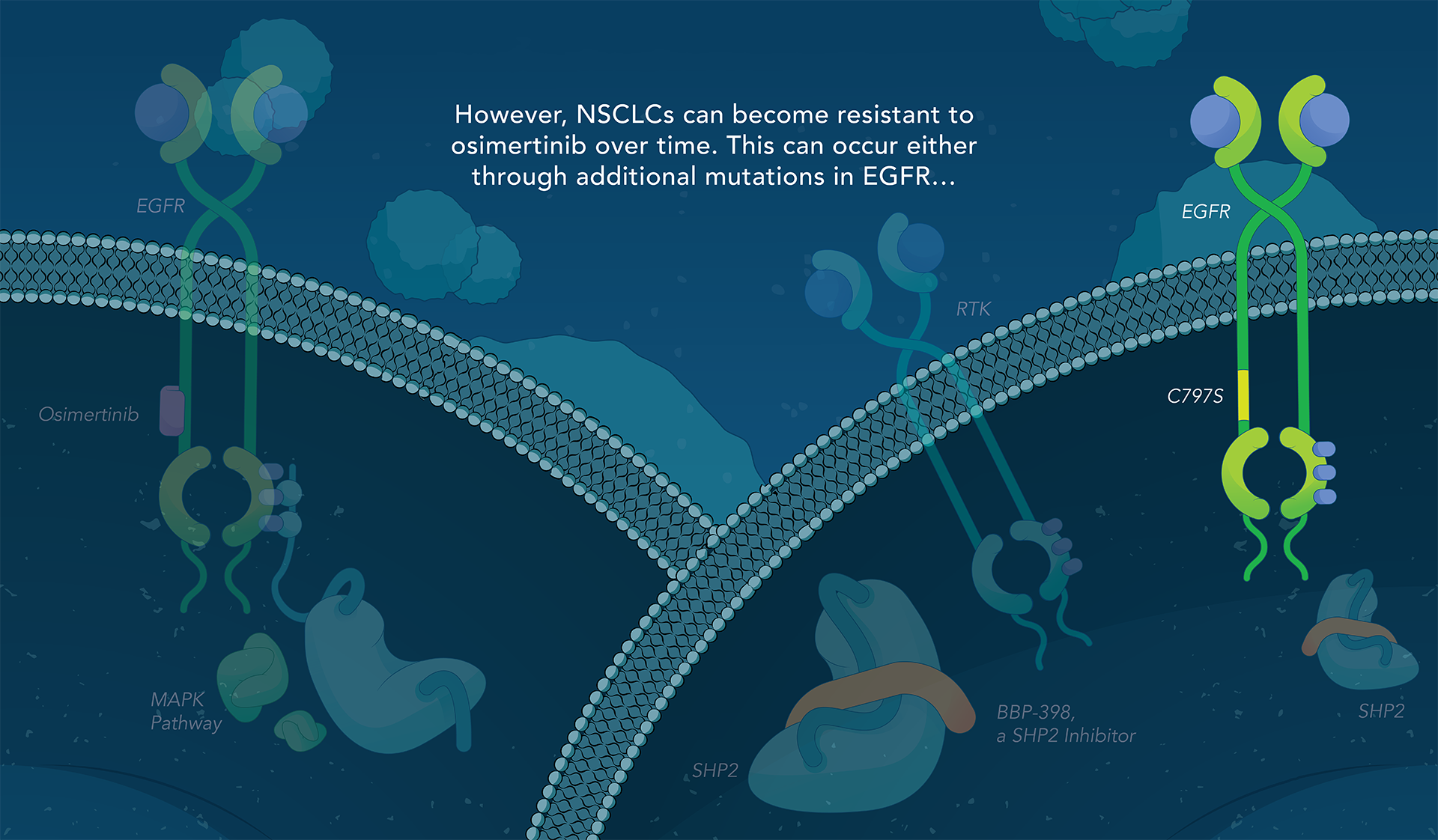
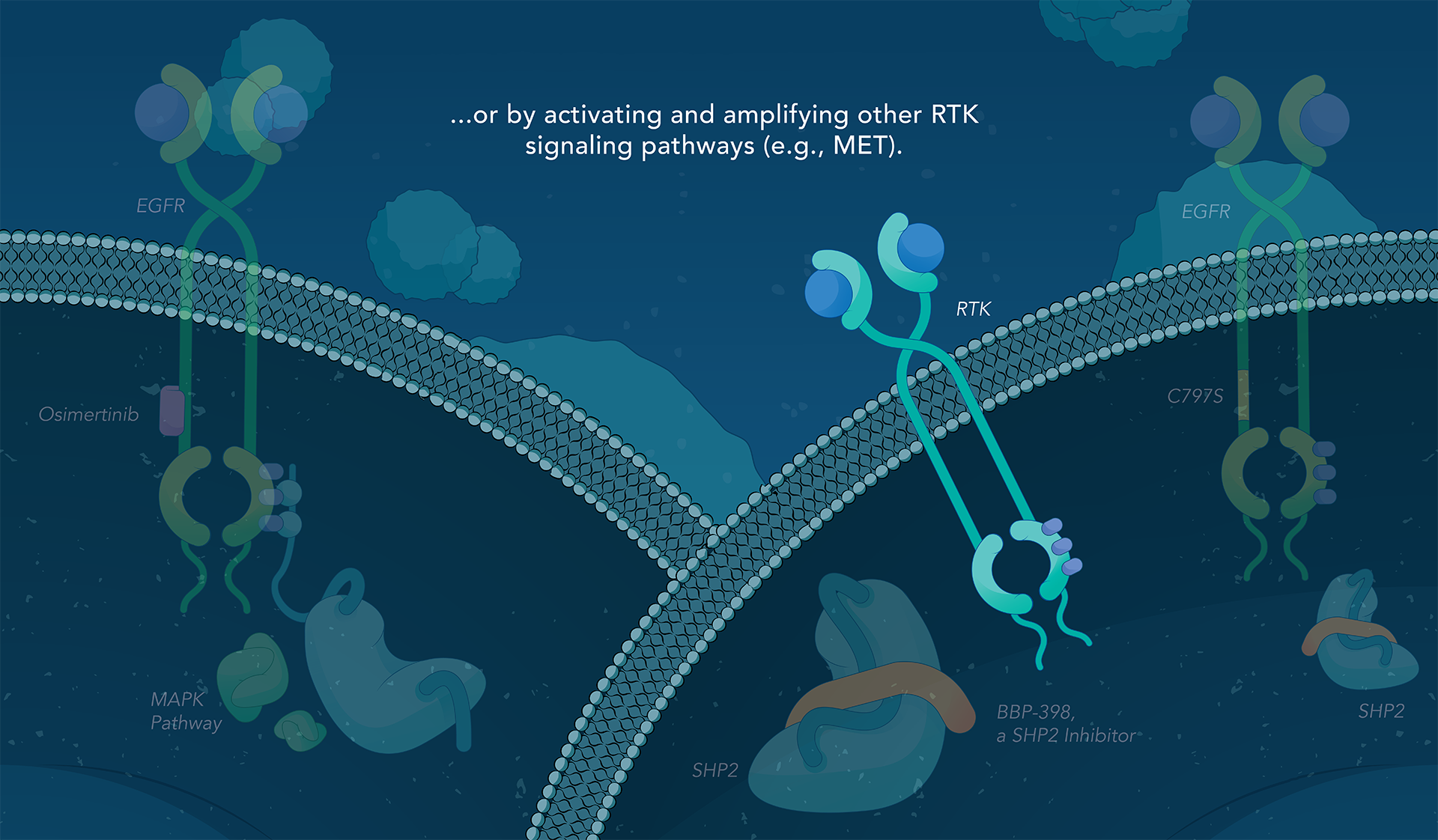
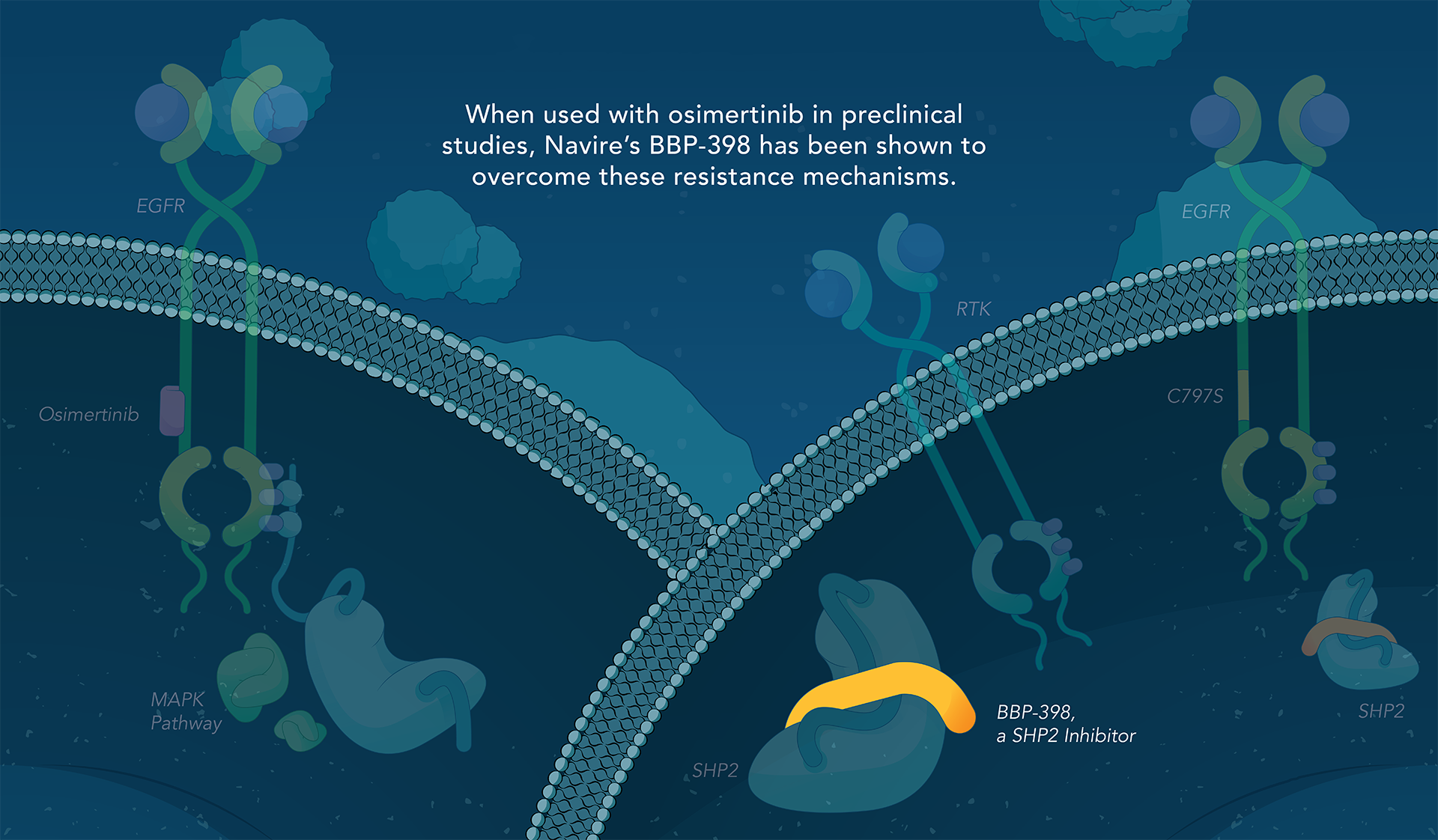
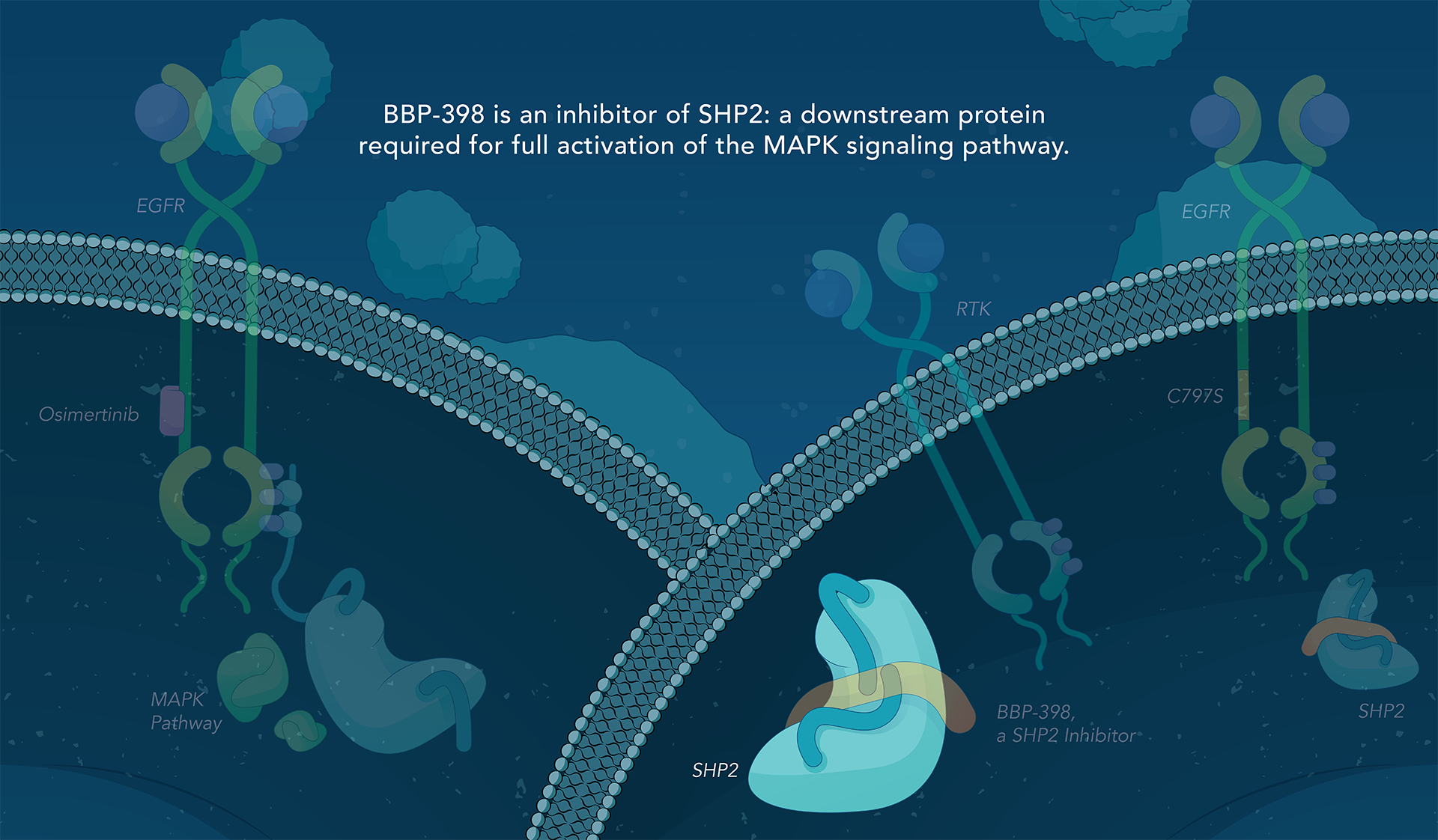
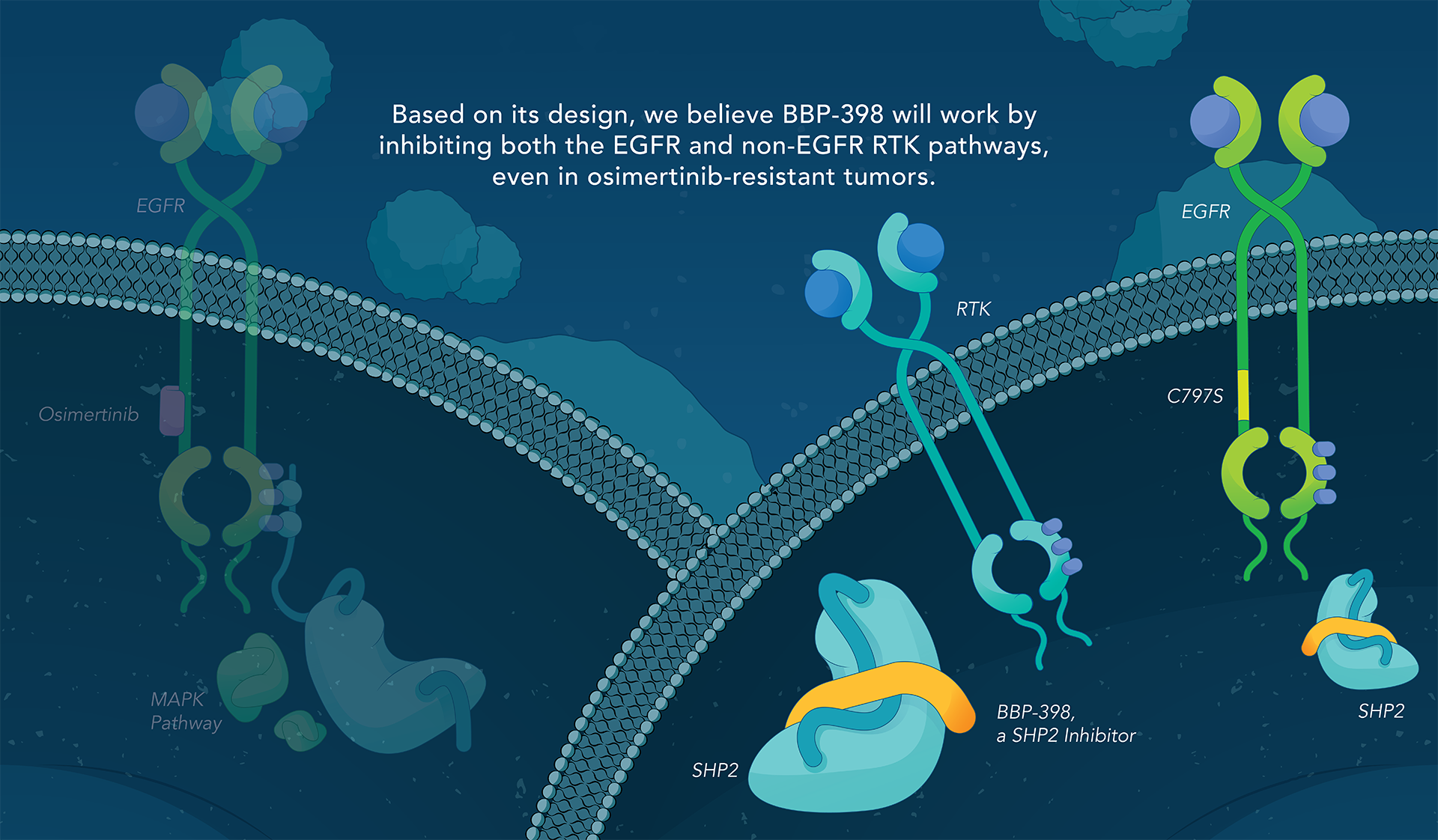
SHP2 (Src homology region 2-containing protein tyrosine phosphatase, encoded by the PTPN11 gene) is a protein-tyrosine phosphatase that links growth factor, cytokine and integrin signalling with the downstream RAS/ERK MAPK pathway to regulate cellular proliferation and survival. Overactivity of this pathway — often driven by distinct genetic mutations — is the fundamental cause of, or a critical contributor to, many forms of cancer, and is a mechanism of resistance to several targeted therapies. Inhibiting SHP2 offers a novel approach to potentially treat tumors driven by this pathway.
Navire Approach
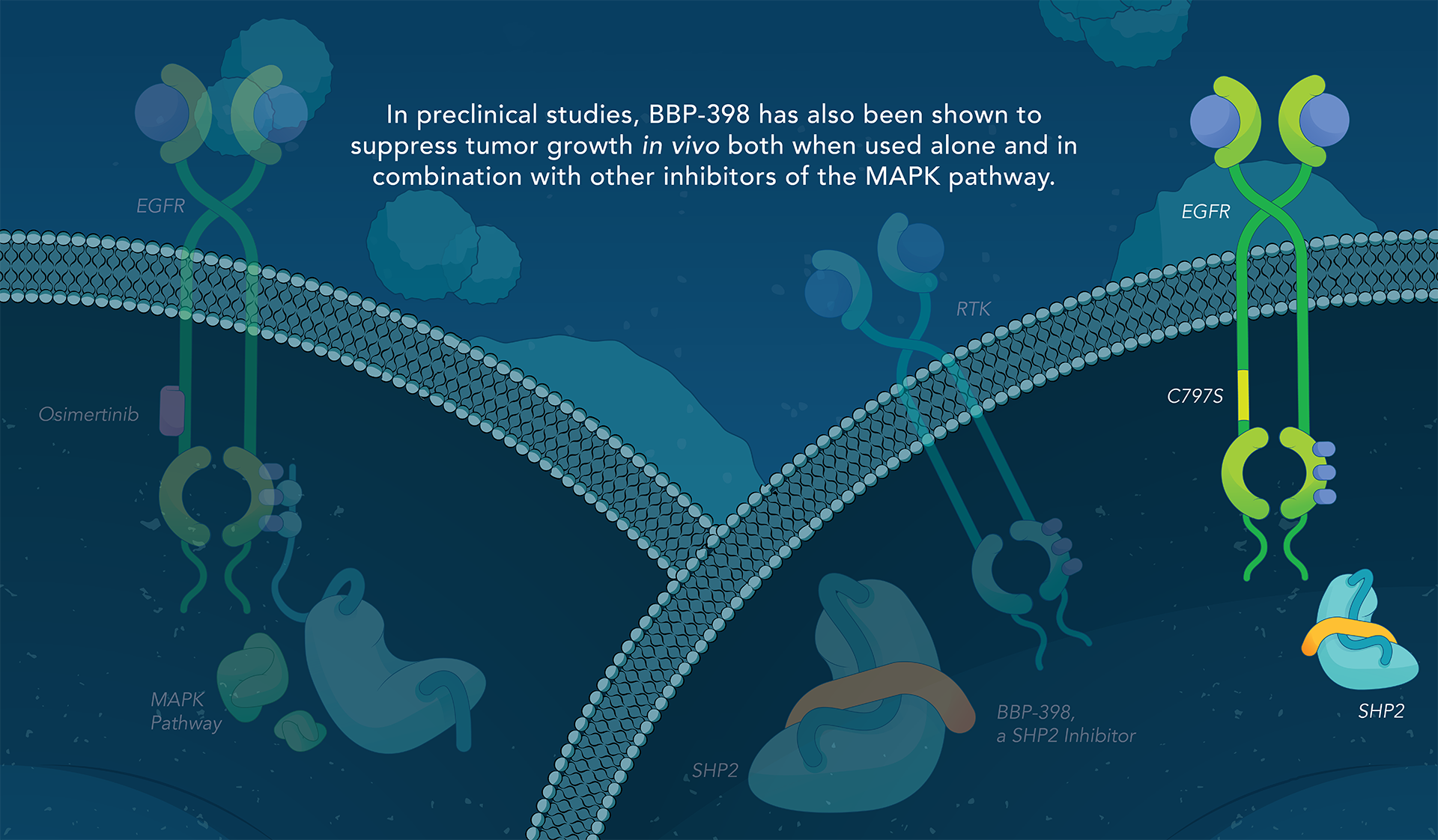
Navire Pharma is developing small molecules to treat cancers driven by increased activity of SHP2-regulated pathways. Navire’s compounds are designed to act as “molecular glue” by binding to inactive SHP2 and preventing activation, blocking its ability to promote tumor growth.
We are advancing BBP-398, a SHP2 inhibitor, in patients with solid tumors driven by mutations in the MAPK signaling pathway, including RAS and receptor tyrosine kinase genes. BBP-398 was developed through a collaboration with The University of Texas MD Anderson Cancer Center’s Therapeutics Discovery division.
BBP-398 is currently being evaluated in multiple clinical studies. For more information about specific studies, see clinicaltrials.gov.
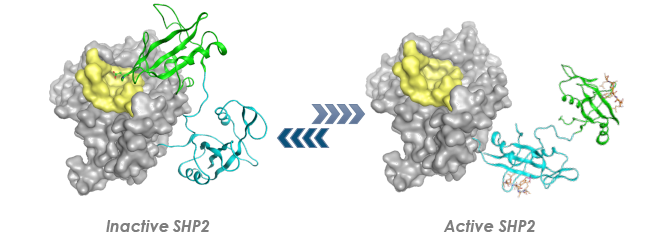
SHP2 Regulation
SHP2 activity is regulated by interaction between its N-terminal SH2 domain and its catalytic (phosphatase) domain. In the inactive form of the protein, the N-terminal SH2 domain blocks the catalytic site, inhibiting phosphatase activity. Upon binding of phosphotyrosyl peptides to the N-SH2 domain, this inhibitory effect is relieved, activating SHP2 signaling.
SHP2 also regulates the adaptive immune response by binding to PD-1 and dephosphorylating CD28 and LCK, thereby suppressing T-cell activity against growing tumors. Inhibiting SHP2 may relieve this negative effect, enhancing the patient’s immune response to fight cancer proliferation.
Publications
About Us
Navire Pharma is harnessing advances in our understanding of SHP2’s role in cancer signaling to develop novel therapies for rare and difficult-to-treat cancers. SHP2 was discovered and characterized by Professor Benjamin Neel, Ph.D., director of the Laura and Isaac Perlmutter Cancer Center at New York University and co-founder of Navire.
Our approach is based on breakthrough discoveries at the Institute for Applied Cancer Science, led by co-founder Phil Jones, Ph.D., at the University of Texas MD Anderson Cancer Center. Together with MD Anderson, BridgeBio launched Navire Pharma in 2017 to create new drugs based on these scientific advances.
In May 2022, we entered an exclusive license with Bristol Myers Squibb to develop and commercialize BBP-398 in oncology worldwide, except for in mainland China and other Asian markets, which are part of our separate strategic collaboration with LianBio announced in 2020. Additionally, we have a non-exclusive clinical collaboration and supply agreement with Amgen to evaluate the combination of BBP-398 with LUMAKRAS® (sotorasib) in patients with advanced solid tumors with the KRAS G12C mutation.
Navire’s team of veteran biotechnology executives are partnering with leading cancer experts to advance effective therapies to patients as rapidly as possible.
Navire is a member of the BridgeBio family
BridgeBio is a team of experienced drug discoverers, developers and innovators working to create life-altering medicines that target well-characterized genetic diseases at their source. BridgeBio was founded in 2015 to identify and advance transformative medicines to treat patients who suffer from Mendelian diseases, which are diseases that arise from defects in a single gene, and cancers with clear genetic drivers. BridgeBio’s pipeline of over 20 development programs includes product candidates ranging from early discovery to late-stage development.